Interface your LIS with BloodNet
To save time and reduce keying errors, you can interface your facility's laboratory information system (LIS) with BloodNet.
Interfacing your LIS means you'll have easy access to near real-time blood inventory and stock movement information.
A BloodNet-to-LIS interface saves you significant processing time because you don't need to duplicate stock data into your LIS after you've entered it into BloodNet. BloodNet will update your LIS automatically, saving time and reducing keying errors. Many facilities have cut down the time it takes to order stock and manage their inventory by more than 90%.
When you interface with BloodNet, your LIS can:
- receipt stock and add unit details into BloodNet when orders arrive from Australian Red Cross Lifeblood(Opens in a new tab/window) (Lifeblood)
- automatically update inventory numbers in BloodNet, so you can see near real-time stock levels when ordering
- upload your unit transfer, discard or transfusion data to BloodNet.
Interfacing with BloodNet also increases the speed and quality of data transfer between blood sector stakeholders.
This helps us improve:
- supply planning
- budget development
- usage reporting
- data analysis.
The BloodNet interface data flow diagram shows how the interface works to help medical officers, facility staff and suppliers.
Find out more about BloodNet.
BloodSTAR integration
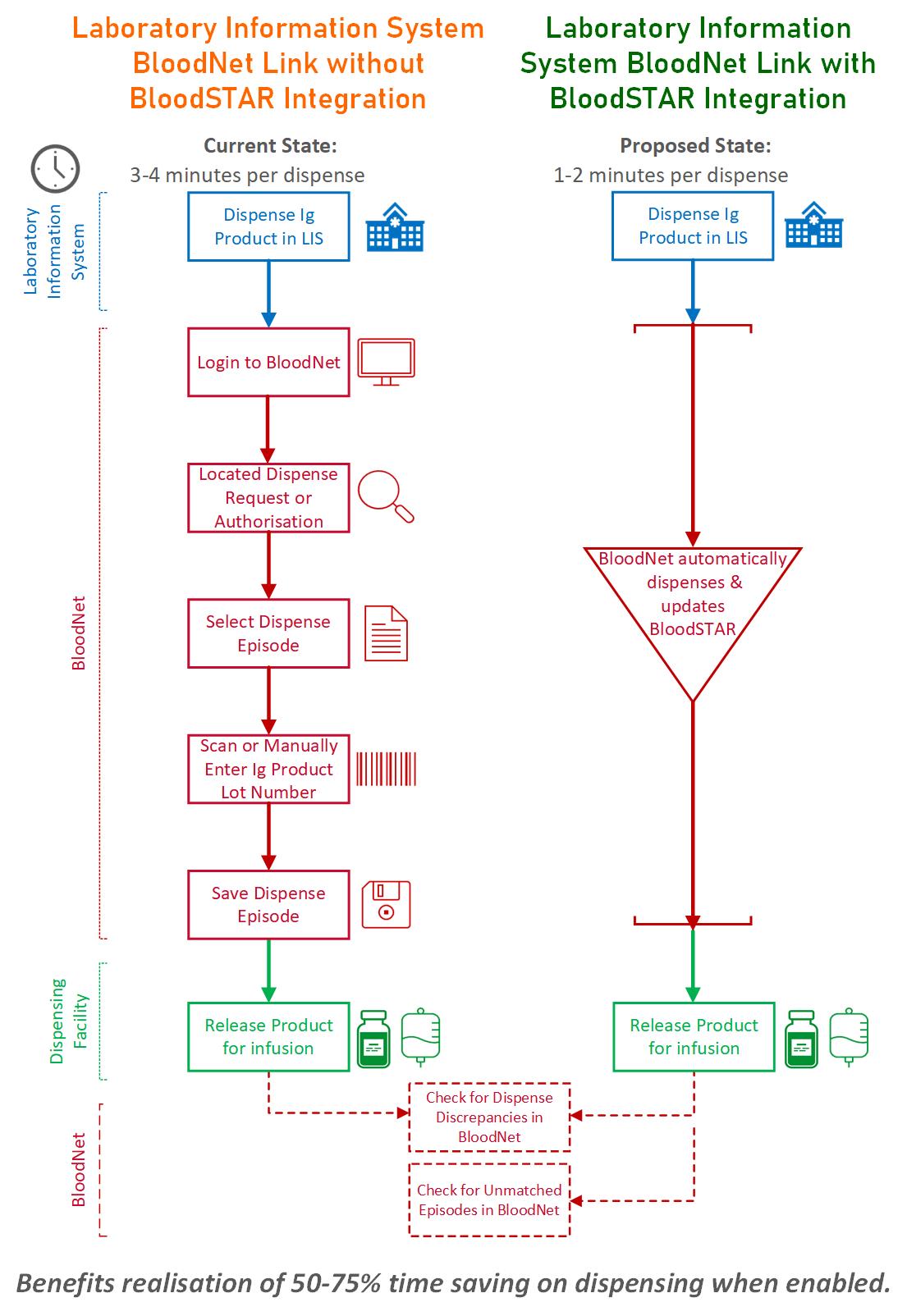
The BloodSTAR integration allows you to transfer immunoglobulin (Ig) dispense data from your LIS into BloodNet and BloodSTAR. This means you can dispense Ig products almost entirely within your LIS because BloodNet will automatically dispense and update BloodSTAR.
It also means checks to resolve dispense discrepancies or unmatched episodes will be much quicker.
Adding a BloodSTAR integration to your BloodNet-to-LIS interface can save up to 75% of your time for each dispensing process.
This diagram shows the benefits and potential time savings when you enable our BloodSTAR integration.
Find out more about BloodSTAR.
BloodSTAR integration for new interfaces
We include BloodSTAR integration when you set up your BloodNet-to-LIS interface. There are additional BloodSTAR verification steps as part of your testing.
BloodSTAR integration for existing interfaces
If you already have a BloodNet-to-LIS interface and you want to enable the BloodSTAR integration, please email support@blood.gov.au or call 1300 025 663.
Certified LIS vendors
To ensure all BloodNet-to-LIS interfaces operate correctly, we have a LIS verification and certification process. This makes sure all LIS interfaces can communicate as expected with our BloodNet system.
All certified vendors have verified and tested their BloodNet-to-LIS interfaces to ensure their facility integration works.
Only certified vendors can use our BloodNet–LIS interface certified logo.

These LIS vendors have completed our LIS interface certification.
| LIS vendor | Version | Notes |
| eBlood | v3.3 | This certification is subject to two exceptions for UnitFate Codes 7 'Reserve' and 9 `Convert’. Separately not sending ‘FatedBy’ data. |
| AUSLAB | vAUSLAB-2017-Q1 | This certification is subject to two exceptions for UnitFate Codes 7 'Reserve' and 8 'SentTo'. |
| BloodTrack | v4.10 | – |
| Cirdan ULTRA | V4.6.16 | This certification is subject to four exceptions for UnitFate Codes 6 `Assumed Transfused’, 7 'Reserve', 8 `SentTo’ and 9 `Convert’. |
| TrakCare Laboratory | L2012 v3.3 | – |
| SoftBank | v 25.7.0.0 | This certification is subject to three exceptions for UnitFate Codes 7 'Reserve' and 8 'SentTo' and 9 'Convert'. Separately UnitFate Code 6 'AssumedTransfused' also only runs once per day and does not poll regularly in real time. |
| EVOLUTION | v5 | – |
| Oracle Millennium | v2018.11.07 | This certification is subject to three exceptions for UnitFate Codes 6 `Assumed Transfused’, 8 `SentTo’ and 9 `Convert’. |
Technical specification for interfacing
Our technical specification covers the technical details for each data element of our BloodNet-to-LIS interface.
All types of LIS software must use this specification.
LIS vendors should refer to our technical specification when setting up, testing and verifying their systems.
Certifying your LIS interface
It's vital that all BloodNet to LIS interfaces operate correctly. If you're a LIS vendor, you must pass our vendor and facility certification before we can enable your interface to operate.
To pass the certification, you must verify that your LIS will work as expected in the production environment.
Once you pass our certification, we'll issue production credentials. Then you can enable your BloodNet-to-LIS interface to operate in your client's facility.
Verifying your LIS
Before we allow your LIS interface to operate in a production environment, we need to verify it in our test environments.
There are 2 stages to verifying your LIS:
- Vendor verification
- Facility verification
With vendor verification, you'll test your LIS interface in our vendor BloodNet system integration testing (SIT) environment to ensure it communicates as expected with our BloodNet LIS web services.
Once you've successfully tested your LIS at the vendor level, you'll test it again in a facility BloodNet user acceptance testing (UAT) environment to ensure it still communicates as expected with our BloodNet LIS web services.
We must confirm and certify your vendor verification before you can begin testing in the facility BloodNet UAT environment.
Before you can test and verify your LIS, you must:
- Have each new LIS administrator create their user account in BloodPortal(Opens in a new tab/window).
- Provide written approval from every Australian Health Provider (AHP) whose data will be accessible in BloodNet.
- Fill out and submit the LIS interface sign-up form with your AHP approvals.
Once you've completed these steps and we've approved your vendor BloodNet SIT environment access, you can start your vendor verification process.
Stage 1: Vendor verification
The vendor BloodNet SIT environment is: https://sit.blood.gov.au/BloodNet5/LISService/LISService.svc(Opens in a new tab/window)
The corresponding BloodNet website is: https://sit.blood.gov.au/BloodNet5/Web(Opens in a new tab/window)
The vendor BloodNet SIT environment is available 24 hours a day, 7 days a week. Our support hours for test environments are Monday to Friday from 9:00 am to 5:00 pm Canberra time.
If we plan any changes or outages to the test environment, we'll email all LIS administrators, developers and designated contact officers.
Stage 2: Facility verification
The facility BloodNet UAT environment is: https://uat.blood.gov.au/BloodNet5/LISService/LISService.svc(Opens in a new tab/window)
The corresponding BloodNet website for user acceptance testing is: https://uat.blood.gov.au/BloodNet5/Web(Opens in a new tab/window)
The facility BloodNet UAT environment is available 24 hours a day, 7 days a week. Our support hours for test environments are Monday to Friday from 9:00 am to 5:00 pm Canberra time.
If we plan any changes or outages to the test environment, we'll email all LIS administrators, developers and designated contact officers.
Verification test scenarios
For both vendor and facility verification, you need to run the following scenario testing (Scenarios A, B and C).
After testing in the vendor BloodNet SIT environment, you must send us your report for us to confirm and verify.
You can't begin testing in the facility BloodNet UAT environment until we've certified your vendor verification tests.
After testing in the facility BloodNet UAT environment, you must send us your report for us to confirm and verify.
Scenario A: Acknowledging issue notes
In this scenario, the facility will acknowledge some receipted issue notes.
- The facility will call the GetReceiptedIssueNotes web service method to return a list of receipted issue notes awaiting acknowledgement.
- The facility then calls the AcknowledgeReceiptedIssueNotes web service method to acknowledge the returned issue notes (from the GetReceiptedIssueNotes).
You can verify the scenario when the:
- web service method calls are successful
- LIS log page on the BloodNet website lists the acknowledged issue notes
- information on the receipting web page shows the acknowledged issue notes.
We'll then confirm these results on at least 5 calls and 50 issue notes.
Scenario B: Update inventory levels
In this scenario, the facility will send up-to-date information on its stock levels.
- The facility will call the UpdateRealTimeInventoryLevels web service method with its stock levels.
You can verify the scenario when the:
- web service method call is successful
- LIS log page on the BloodNet website lists the stock update details
- inventory update shows on the LIS inventory dashboard web page.
We'll then confirm these results for a minimum of 24 hours of 15-minute interval LIS inventory level updates.
Scenario C: Fate of unit
In this scenario, the facility will send a discard fate for a component and product.
Step 1: The facility will call the FateOfUnit web service method with a component discard payload.
You can verify the scenario when the:
- web service method call is successful
- LIS log page on the BloodNet website lists the fate details
- fate summary page reflects the new component discard
- user is also able to view the component discard fate details.
Note: You must repeat Scenario C for each of the UnitFate types your system uses against each of the product types your facility orders. For example, if you have 10 potential UnitFate types and you order red blood cells, platelets, frozen and immunoglobulin products, you need to test at least 40 individual scenarios. One for each UnitFate type with each product type.
We'll then confirm these results on at least 40 calls and 40 successful fate records.
Useful documents
Use these documents to help guide your verification testing process.
Production credentials
Once your interface passes our vendor and facility certification, you'll get your LIS production service account and credentials.
If you need to add new users for the production environment, please:
- Have each new LIS administrator create their user account in BloodPortal(Opens in a new tab/window).
- Update and re-submit the LIS interface sign-up form.
Don't create new user accounts for anyone already registered on BloodPortal for the LIS testing and verification stages.
Interface updates
These LIS interfaces are in process at various stages of completion.
| LIS | Interface status |
| Apollo (Sonic Healthcare)(Opens in a new tab/window) | Currently under development |
| AUSLAB (Magentus)(Opens in a new tab/window) | In production |
| BloodTrack (Haemonetics)(Opens in a new tab/window) | Available for implementation |
| e-Blood (Magentus)(Opens in a new tab/window) | In production |
| EVOLUTION vLab (Magentus)(Opens in a new tab/window) | In production |
| MediPATH (LRS Health)(Opens in a new tab/window) | Awaiting quote from vendor |
| Millennium (Oracle)(Opens in a new tab/window) | In production |
| Pathology Laboratory System (Kestral)(Opens in a new tab/window) | Currently under development |
| SoftBank (SCC Soft Computer)(Opens in a new tab/window) | In production |
| TrakCare Laboratory (InterSystems)(Opens in a new tab/window) | In production |
| Ultra (Cirdan)(Opens in a new tab/window) | Available for implementation |
Get in touch
For LIS interface support or questions, contact our Blood Operations Centre.
Our team is available 24/7 for live BloodNet-to-LIS interface support.
Phone: 1300 025 663 (13 000 BLOOD)
Email: support@blood.gov.au
Last updated: 27 Mar 2024
