In this Summer 2019 issue:
- BloodNet Milestones
- BloodNet News
- Immunoglobulin Governance Update
- Imported immunoglobulin product supply contracts
- User Tips
- NBA Christmas Shutdown
BloodNet Milestones
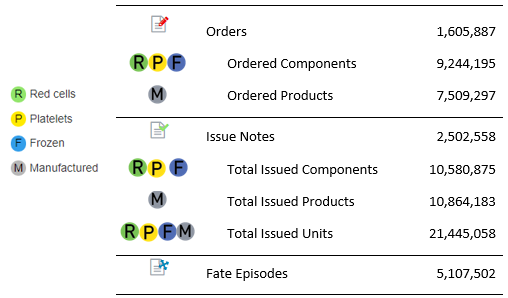
At the National Blood Authority (NBA) we acknowledge that the success of BloodNet would not have been possible without the involvement of the system’s users - here is the latest update to our amazing statistics.
First off the rank is the Royal Brisbane and Women’s Hospital Pathology Laboratory who receipted the 20 millionth product and component on 28 July 2019, followed by the Townsville Laboratory creating the 5 millionth Stock Movement episode on 11 October 2019.
Congratulations on helping us achieve these significant milestones!
BloodNet News
On Sunday 13 October 2019, the National Blood Authority (NBA) successfully launched BloodNet 5.3 into production. Version 5.3 provided a number of beneficial enhancements to improve usability of BloodNet. A copy of the BloodNet 5.3 release notes can be obtained from your Facility Administrator.
Immunoglobulin Governance Update
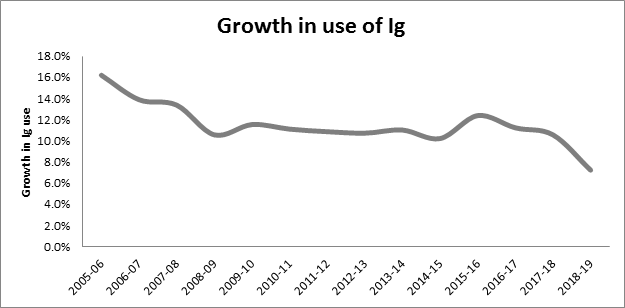
Growth in immunoglobulin (Ig) use is slowing following national implementation of BloodSTAR
The end of year reconciliation for 2018-19 has confirmed that the full year on year growth of Ig demand from 2017-18 to 2018-19 was 7.2%. This is the lowest annual rate of increase since Australia first secured supply sufficiency through national importation of Ig by the NBA in 2004-05. This compares with a budgeted forecast growth of 11.6%, a reduction of 301 kg.
This outcome follows completion of the development and implementation phase of the Ig Governance Program in October 2018, with the full national roll out of BloodSTAR and the commencement of the Criteria Version 3.
Monthly Immunoglobulin data and statistics available now
The NBA is working to establish regular reporting to support the Immunoglobulin Governance Program activities. As part of this, the NBA is reporting some high level statistics regarding immunoglobulin use on its website each month. The Ig Usage Data and Statistics page will display current information across a variety of parameters related to the use of immunoglobulin. The latest Ig data Usage and Statistics data is available under the Best Practice section of our website.
NPS MedicineWise seeking your views on improving the prescription and use of immunoglobulin
NPS MedicineWise is a not-for-profit organisation that works nationally to improve quality use of medicines, medical tests and health technologies. A consortium led by NPS MedicineWise has been engaged by the Commonwealth Department of Health to develop and deliver a program that supports a viable and sustainable immunoglobulin prescribing system. The ‘Value in Prescribing: Immunoglobulin Products’ program is a three-year program that will involve working with the National Blood Authority to promote quality use of immunoglobulin products.
NPS MedicineWise is in the early stages of planning and designing the program and is embarking on a consultation process with experts to identify the priority issues that the program can potentially address. Your experience and insights into use of immunoglobulin products would be highly valuable in guiding this program. For further information, please email IgGovernance@blood.gov.au.
Imported immunoglobulin product supply contracts
The NBA’s contracts with CSL Behring and Grifols Australia for the supply of imported immunoglobulin products have been extended until 31 December 2020.
Under the national blood arrangements, Grifols Australia will supply the IVIg product Gamunex 10% from 1 November 2019. The availability of this additional product will enhance the security of imported immunoglobulin supply arrangements over the remaining period of the contracts. Implementation arrangements for Gamunex 10% included the dissemination of appropriate communications and support materials to stakeholders including the Australian Red Cross Lifeblood, approved health providers, clinicians and patients.
User Tips
Bulk Dispensing of SCIg
The NBA 5.3 release introduced the functionality to dispense multiple subcutaneous immunoglobulin (SCIg) doses in one dispense episode. The process on dispensing multiple SCIg doses in one dispense episode is available in the ‘Bulk Dispense of SCIg’ tip sheet.
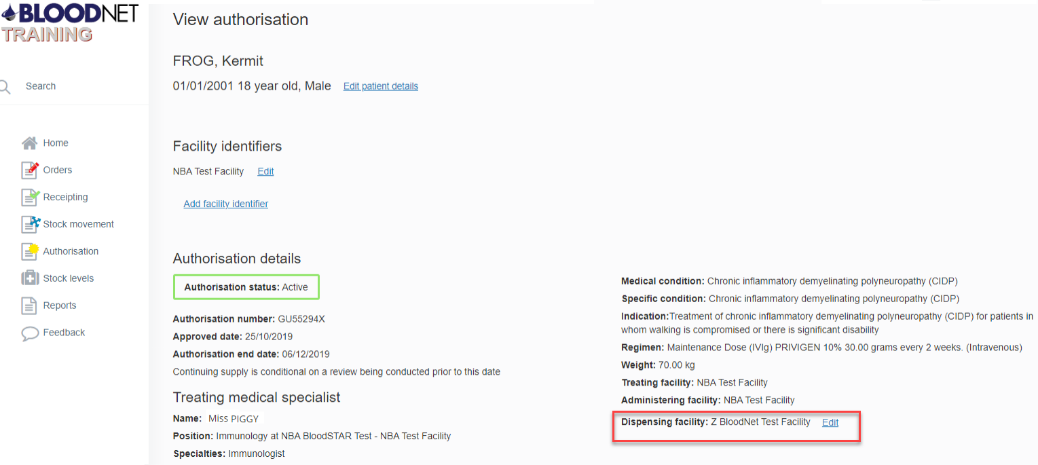
Updating a Dispensing Facility on a Patient Record
If a patient has permanently moved to your facility, you may be required to update their dispensing facility recorded in BloodNet. This can be updated through the patient’s record.
- Click on the ‘Authorisation’ tile from the BloodNet homepage
- Conduct a national search by entering in either the patient’s authorisation number, or at least three patient identifiers e.g. surname, first name and date of birth
- From the patient record, click ‘Edit’ against the recorded dispensing facility
NBA Christmas Shutdown
Please note that the NBA office will be closed from 5pm AEDT on 24 December 2019 until 8am AEDT 2 January 2020.
For emergency system support you are able to contact 1300 0BLOOD (1300 025663). For all other non-urgent enquiries, please email support@blood.gov.au.
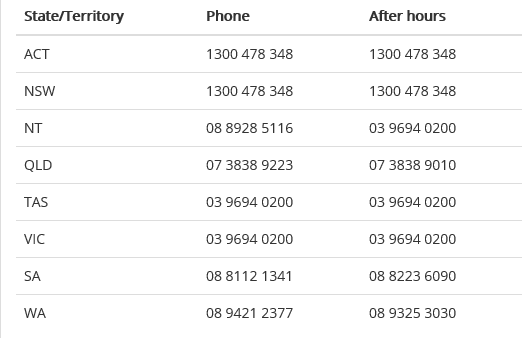
For clinical enquiries please contact the Australian Red Cross Lifeblood on the following numbers:
For further information
Further information on BloodNet is available online at https://www.blood.gov.au/bloodnet. We welcome feedback and suggestions on how we can improve this newsletter. If you have any topics you would like included in future newsletters, please let us know by calling 1300 0BLOOD (1300 025663) or by sending an email to support@blood.gov.au