The NBA exists to give effect to national arrangements agreed and funded by all Australian governments that save and improve lives in Australia through a world-class blood supply.
Corporate Governance
Four committees assist the NBA Chief Executive with the corporate governance and administration of the agency.
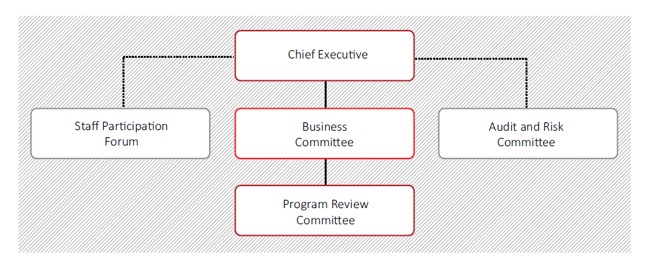
Business Committee
The NBA Business Committee is the primary governance mechanism for the NBA. It provides strategic oversight and direction for the overall management of the NBA and its business, finance, technology, audit, risk and corporate activities.
Program Review Committee
The Program Review Committee systematically reviews the implementation and delivery of individual program commitments and assesses the priorities, progress and outlook for program work.
Audit And Risk Committee
The committee usually comprises three members external to the NBA who are appointed by the NBA Chief Executive. Members have expertise to provide independent advice and assurance to the Chief Executive on strategies to enhance the organisation’s governance control and risk management framework, assist with planning and conducting the NBA internal audit program and support financial and legislative compliance.
![]() Audit and Risk Committee Charter (157.77 KB)
Audit and Risk Committee Charter (157.77 KB)
![]() Audit and Risk Committee Charter (221.4 KB)
Audit and Risk Committee Charter (221.4 KB)
Staff Participation Forum
The forum is established under the NBA Enterprise Agreement to provide a formal mechanism for NBA management to consult directly with employee representatives about significant issues relating to employment matters. The forum comprises NBA staff representatives, NBA management representatives and a Work Health and Safety representative.
National Blood Sector Governance Arrangements
The NBA is a statutory body and portfolio agency of the Commonwealth Department of Health and Aged Care. Funding for the national blood arrangements is jointly provided by all Australian governments, with the Commonwealth Government providing 63 per cent of funding and states and territories 37 per cent.
The National Blood Agreement signed by all Australian governments in 2002 outlines the policy framework for the national blood arrangements. The agreement outlines the:
- nationally agreed objectives of governments for the blood sector
- governance arrangements for the sector
- administrative arrangements for the management of the national blood supply
- financial arrangements for the national blood supply.
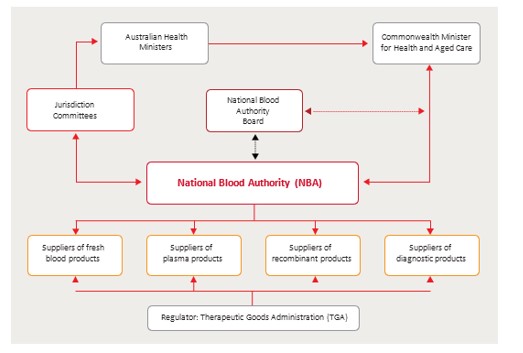
The key governing arrangements within the Australian blood sector
NBA Board
The NBA Board is established under the NBA Act with functions that mainly involve consideration of strategic blood sector issues, and advice to the NBA Chief Executive about the discharge of the NBA’s functions. The Board is not a decision-making body and has no formal or direct role in the governance or day-to-day management of the NBA.
More information on the Board and its Members can be found on the Board Page.
Jurisdictional Blood Committee (JBC)
The National Blood Agreement established a Jurisdictional Blood Committee (JBC) as a committee of senior government officials with representation from the Commonwealth, State and Territory governments.
The JBC represents jurisdictional positions on policy, demand, funding and evidence-based approaches to emerging products, services and technologies in relation to blood, blood products and blood services.
The JBC has an important role in engaging with the NBA to develop annual supply and demand planning and budgeting to ensure an adequate and affordable supply of blood and blood related products that meet clinical demand. The National Supply Plan and Budget (NSP&B) is developed and agreed each year with governments.
The NSP&B estimates the volumes and types of products required from suppliers following consultations with clinicians, jurisdictions and patient groups and budgets are developed accordingly. The NSP&B is approved by all Health Ministers on an annual basis.
Current membership of the JBC is as follows:
Further information is available from the JBC Secretariat by email jbc@blood.gov.au.
Statutory Committees
Four committees have been established by the NBA Chief Executive under section 38 of the National Blood Authority Act 2003 to provide advice and assist with the performance of the NBA’s functions.
The terms of reference, membership details and section 38 instruments for these committees are available on the NBA website (link). Their roles and functions are as follows.
National Immunoglobulin Governance Committee
The National Immunoglobulin Governance Committee (NIGAC) provides clinical expertise and advice to assist the work of the NBA’s Immunoglobulin Governance Program. Members are appointed based on expertise and experience.
The NIGAC is chaired by Emeritus Professor Robert Moulds. Its members represent medical specialisations, consumer advocacy, epidemiology, health economics, nursing, large and small jurisdictions, Australian Red Cross Lifeblood and the NBA.
The NIGAC is supported by the specialist working groups for immunology, haematology, neurology and transplantation medicine.
Australian Bleeding Disorders Registry Steering Committee
The Australian Bleeding Disorders Registry (ABDR) is used daily by clinicians to help manage the treatment of people with bleeding disorders and to understand more about the incidence and prevalence of bleeding disorders.
The ABDR Steering Committee provides advice to the NBA on the governance and use of the ABDR. It consists of representatives involved in clinical management, advocacy, and funding of treatment for people with bleeding disorders. The committee chair is Dr Chris Barnes.
Patient Blood Management Advisory Committee
The Patient Blood Management Advisory Committee (PBMAC) provides advice and guidance to the NBA about implementing patient blood management in Australia, including developing a new National Patient Blood Management Implementation Strategy.
The PBMAC was established in 2019, replacing the previous Patient Blood Management Steering Committee. Its members have expertise and knowledge in the health sector, blood management, education, quality and safety, and consumer issues.
Haemovigilance Advisory Committee
The NBA’s national Haemovigilance Program is informed by this expert advisory committee. It provides advice and guidance to the NBA on adverse event reporting originating from health service organisations, on national transfusion safety priorities and on the development and implementation of the strategic framework.
Australian Health Ministers’ Advisory Council
The Australian Health Ministers’ Advisory Council (AHMAC) provides support to the COAG Health Council. It advises the health ministers on strategic matters relating to the coordination of health services across the nation and, as necessary, with New Zealand. The Council considers blood sector matters referred to it by the Jurisdictional Blood Committee (JBC) through the Hospitals Principal Committee, and reports as necessary to the COAG Health Council. The Council has no statutory power and decisions are reached by consensus.
Hospitals Principal Committee
The Hospitals Principal Committee (HPC) considers, and provides advice to the Australian Health Ministers’ Advisory Council (AHMAC), where appropriate on:
- all activities which largely relate to hospital care including emergency departments, outpatient care, inpatient care and alternatives to hospital care
- implementation of the health reform agenda as it applies to hospital care
- clinical, technical and medico–ethical developments
- the appropriateness, likely impact, policy implications, effectiveness and safety of clinical and technical developments.
Jurisdictional Blood Committee
Australian, state and territory governments are represented on the Jurisdictional Blood Committee (JBC), which was established by the National Blood Agreement in 2003. The JBC is the conduit between governments and the NBA. It represents the Australian, state and territory governments’ positions on blood policy, demand, supply planning and product distribution, funding and evidence-based approaches to emerging products, services and technologies. It oversees the NBA’s role in blood supply contracting. It is also the primary body responsible for providing advice and support on these matters to the COAG Health Council through the HPC and the AHMAC. For details on the current membership of JBC visit the Jurisdictional Blood Committee page.
NBA Board
Established under the National Blood Authority Act 2003, the National Blood Authority (NBA) Board provides advice to the General Manager about the performance of the NBA's functions. The Board is not a decision making body and has no formal or direct role in the governance or management of the NBA. The Board does however, consider key strategic issues facing the NBA. For more information on the membership of the Board visit the National Blood Authority Board page.
Stakeholders
Australian, state and territory governments
As signatories to the National Blood Agreement, the Australian, state and territory governments are responsible for:
- establishing the policy framework and specific policies relating to the national blood supply
- overseeing the NBA’s management of the blood supply arrangements
- fostering the development and implementation of best-practice systems to promote efficient use and minimal wastage of blood and blood products
- providing information on demand for blood and blood products
- managing local issues.
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the regulator for blood and blood products in Australia. It is responsible for:
- regulating the efficacy and safety of blood and blood products under the Therapeutic Goods Act 1989
- auditing supplies against good manufacturing practice
- issuing product recalls
- issuing modifications to safety standards
- issuing directives such as those relating to donor deferral.
Suppliers of blood and blood products in Australia
The NBA contracts with a number of suppliers for the provision of blood and blood products including:
- the Lifeblood which collects fresh blood from voluntary donors
- CSL Limited, which fractionates plasma from blood collected by the Lifeblood and supplies a range of plasma products purchased through the NBA under the National Fractionation Agreement for Australia.
Australia is reliant on an imported supply of plasma derived Factors XI and XIII, Anti-inhibitor Coagulant Complex Concentrates, Protein C, a plasma-derived Rh(D) immunoglobulin products, Fibrinogen Concentrate and C1 Esterase Inhibitor Concentrate, which are not manufactured in Australia. A number of recombinant clotting factor products and immunoglobulin products are also imported. These products are not manufactured in Australia. Current contracts for imported plasma and recombinant products can be found here.

